The availability of internet-based pornography, especially among young adults, has raised concerns about its impact on health. There are no standardised diagnostic criteria for pornography addiction. Excessive internet pornography use is a new form of behavioural addiction, which differs from compulsive sexual behaviour disorder, as it is classified as an impulse control disorder rather than an addictive condition in the ICD-11 (International Classification of Diseases for Mortality and Morbidity Statistics Eleventh Revision). Pornography addiction is not officially recognised as a disease due to ongoing debates among researchers about its definition and nature. The study aimed to fill this gap by applying adapted DSM-5 (Diagnostic and Statistical Manual of Mental Disorders [Fifth Edition]) criteria to assess “pornography-watching disorder” (PWD).
The study aimed to deepen the understanding of PWD as a behavioural addiction and apply DSM-5 criteria to assess PWD. The findings emphasise the need for standardised diagnostic tools for PWD and propose targeted interventions, especially for high-risk groups. These results add to the ongoing debate about whether pornography addiction should be recognised as a distinct behavioural disorder.
https://www.jmir.org/2025/1/e49860
Ethical Issues
Latest Pornography statistics
According to various research Centres:
• 61% of the general population, ages 13-65, consume pornography
• 78% of men consume pornography
• 44% of women consume pornography
• 68% of 18–29-year-olds consume pornography in China
• 61% of Pornhub traffic comes from ages 18-34.
• 76.5% of students had accessed online sexual entertainment
• 97% of male and 36% of female Japanese students consumed pornography
• 95.9% of men and 59.1% of college students in the Czech and Slovak Republics consumed porn, (71.8% total)
• 58% of teens stumble on porn accidentally
• Most teens who view porn have been exposed to violent porn
• 67% of pastors have a personal history with pornography, while 18% of pastors in the United States currently struggle with pornography
• 52% of women view porn as a negative in their relationships
Download the full report from:
Total porn ban foreseen for the future
A UK poll revealed that 55% of UK adults support banning VPNs for under-18s. Some USA legislators intend to implement similar measures.
https://www.forbes.com/sites/zakdoffman/2025/11/08/porn-ban-google-issues-new-warning-for-all-smartphone-users/
Pornography causes lasting harm
There has been an increase in pornography use due to the rise of new technology.(1) A study done in 2018 found that 91.5% of men and 60.2% of women had consumed it in the previous month.(2) There is a big concern about the damage done to children as young as 10 years who view pornography.(3)
Porn use is detrimental in terms of behaviour, relationships, and mental and physical well-being. They also have trouble reducing or controlling their pornography use.(4)
Common damages done due to porn viewing:
- Pornography reduces grey matter in the brain.(5)
- Porn urges people to pursue content that was previously seen as disgusting(6)
- Relationships suffer(7) (8)
- Less sex in marriage and erectile dysfunction(9) (10)
- Porn viewers engage in rule-breaking and aggressive behaviours(11)
Late-Term Abortions Result In Live Births
• Late-term abortion is an act so heinous, nearly all Americans are opposed to it. What you may not know, however, is the staggering number of late-term abortions that result in a live birth, a fact the abortion industry works tirelessly to conceal.
• For years, abortion advocates have engaged in an aggressive campaign of lies,
attempting to gaslight the public into believing these abortions do not occur. When outright denial fails, they offer morally bankrupt justifications for the killing of viable children in the later stages of pregnancy.
• A recent study, titled Second-Trimester Abortion and Risk of Live Birth, published in the pro-abortion American Journal of Obstetrics and Gynaecologists tells the shocking truth.
• Researchers examined 13,777 abortions performed between 15- and 29-weeks’ gestation and found that a staggering 1,541 resulted in a live birth. That means 11.2% of these babies were born alive—only to be left to die without medical intervention or outright killed.
• We know that nearly 36% of babies born at 22 weeks survive with proper care, and 82% survive at 25… weeks. Many of the babies born alive in these botched abortion attempts could have lived if only they had been provided the life-saving treatment they deserved. Instead, they were abandoned to die. Some babies even survive at 21 weeks.
• The abortion-friendly media plays its part in this nightmare, carefully avoiding stories of premature babies who survive and thrive. They ignore advances in neonatal care that continue to push the limits of viability earlier and earlier, instead perpetuating the lie that these children are not yet human, not yet worthy of protection.
• The reality of late-term abortion is not just a political issue—it is a human rights crisis. If we are to call ourselves a civilized society, we must confront this evil and demand justice for the most vulnerable among us.
Paris – Comments on Criminal Law Amendment Bill to repeal the Sexual Offenses Act.
• We observe that prostitution is neither sex nor work, but constitutes in itself a form of violence against women:
• The repetition of sexual acts without physical desire, but instead experienced as the consequence of financial need, inequality or as an exploitation of vulnerability, constitutes sexual violence in and of itself.
• The vast majority of prostituted persons have suffered from violence, often sexual, before entering prostitution. Most of them are also victims of many forms of violence while in prostitution (physical, verbal, sexual, psychological.
• There is no “free” and “forced” prostitution: in reality, the sexual act obtained by sex buyers is always coerced. Coerced either by physical coercion of traffickers and pimps, or by the socio-economic coercion that pushes the most vulnerable women and girls into prostitutes.
• There will be no equality between women and men as long as men think that they can buy access to women’s bodies. Prostitution is a part of a continuing patriarchal tradition of making women’s bodies available for men’s benefit.
• Prostitution is based on multiple forms of inequalities: men’s domination over women, rich over poor, North over South, majority groups over minorities. Women from minorities, poor, migrant and marginalized groups form nearly the totality of the prostituted persons in prostitution all over the world.
• These realities also apply to South Africa. By reframing prostitution as a form of work, by normalizing the endless exploitation of women’s bodies as an acceptable income alternative, the government is sending an extremely sad message to its most vulnerable population, in particular women: if you are marginalized, we have nothing better to offer than being exploited in prostitution.
Closing the Loophole on Hemp(Press Release)
Media Release
Embargo: Immediate release Enquiries: Doctors For Life
Date: 2 November 2024 Telephone: 032 4815550
USA – Closing the Loophole on Hemp-Derived Cannabis Products
Article contributors: Alyssa F. Harlow, Adam M. Leventhal, Jessica L. Barrington-Trimis, Keck School of Medicine, University of Southern California, Los Angeles.
A recent authoritative article from the USA has exposed a loophole in the American legislation concerning hemp. The general public in South Africa, and to a certain extent medical practitioners, also consider hemp as an innocent drug. DFL would like to make the South African public aware of this loophole that is becoming very relevant to South Africa. The following is a shorted version of the original article (link at bottom)
The USA federal policy loophole allows psychotropic ie, mind-altering or intoxicating, cannabis products to be commercially marketed and sold across the US-including in states where recreational cannabis is not legal.
The Agriculture Improvement Act of 2018, commonly known as the 2018 Farm Bill,1 legalized the growth and sale of hemp. Hemp is defined as a botanical class of the cannabis sativa plant that contains low concentrations of Δ9-tetrahydrocannabinol (Δ9-THC, which is the most well-studied psychotropic cannabis-specific compound [ie, cannabinoid]) and high concentrations of non-psychotropic cannabidiol (CBD).1
However, hemp also contains low concentrations of hundreds of other cannabinoids besides CBD and THC,2 which, until recently, were believed to be present in amounts too small to produce psychotropic effects.
Under the protection of the Farm Bill,1manufacturers can synthesize and sell hemp-derived cannabis products with psychotropic doses of cannabinoids, such as Δ8-THC, ΔO-THC, Δ10-THC, and hexahydrocannabinol, and others.
These hemp-derived cannabis products produce similar psychotropic effects as Δ9-THC and are being sold across the US as vape cartridges, edibles, concentrates (eg, potent extracts), and tinctures (eg, infused liquids). Hemp-derived cannabis products are also being marketed and sold widely in South Africa, all largely unregulated. Clinicians and policy makers should be aware of public health concerns of widely available, psychotropic, hemp-derived cannabis products that are being manufactured and sold with little regulation, leading to potential health and safety risks. There are 6 main concerns:
1) First, unlike traditional state-regulated Δ9-THC cannabis that is sold in medical or recreational dispensaries for adult use, psychotropic, hemp-derived cannabis products are sold online and by retailers (eg, vape and smoke shops, convenience stores, and gas stations). Retailers selling these products do not have the same safeguards in place that state-run cannabis control bureaus have developed to reduce potential harm to consumers.3 For example, there is no established minimum purchasing age for hemp-derived cannabis products. In addition, there are no requirements for hemp-derived products to include warning labels for the presence of THC, or instructions on their packaging regarding appropriate doses. Retailers that sell psychotropic, hemp-derived cannabis products may also sell alcohol and tobacco products like e-cigarettes and cigarettes; this increases the potential for co-use of multiple substances, which can lead to excessive impairment and greater risk of drug dependence.
2) Second, hemp-derived cannabis products have marketing features that may appeal to youth. For example, such products are available as chocolates, gummies, cookies, and brownies and the packaging and advertisements often use bright and colorful designs. In addition, hemp-derived cannabis vape cartridges come in a wide range of sweet and fruity flavors, which increase appeal among youth and young adults. Because of their similarity to candy and food products, accidental exposure by children, adults, and animals is a concern. Between January 1, 2021, and February 28, 2022, national poison control centers received reports of 2362 cases of Δ8-THC exposures, of which 40% involved accidental exposure to Δ8-THC (82% among youth), 70% required evaluation at a health care facility, 8% were admitted to a critical care unit, and 1 pediatric death was reported. 2 Animal poison control centers have also seen an increase in reports of accidental pet exposure to Δ8-THC.2
3)Third, there is no standardized approach or certification process to synthesizing psychotropic, hemp-derived cannabinoids, and such products could contain dangerous and toxic byproducts. Hemp plants naturally contain low concentrations of psychotropic cannabinoids; manufacturers must first extract CBD from hemp, which they then convert to psychotropic cannabinoids through a series of chemical reactions. Independent laboratory tests of legally purchased hemp-derived cannabis products have revealed the presence of toxic heavy metals (eg, lead), residual solvents (eg, acetone), and multiple unidentified compounds with unknown toxicological harms.4 Inhalation of contaminated cannabis vaping products can lead to serious lung injury, as evidenced by the 2019 EVALI (e-cigarette, or vaping, product use-associated lung injury) outbreak.5
4) Fourth, the psychotropic properties of some hemp-derived cannabis products may be less potent than Δ9THC. Because of lower potency, individuals may consume higher volumes of hemp-derived cannabis products than traditional Δ9-THC products, leading to adverse effects such as hallucinations, vomiting, tremor, anxiety, dizziness, confusion, and loss of consciousness.2 It is not uncommon to see gummies with 50 mg of Δ8-THC per serving (compared with 5-10 mg of Δ9-THC in a standard product sold in a dispensary).
In addition, other synthesized THC isomers may be more potent than Δ9-THC. For example, at the same dose, ΔO-THC is considered 3 times stronger6 than traditional Δ9-THC, and products often contain a blend of different hemp-derived THC isomers. Consumers may not be aware of such differences and may be at risk for adverse effects from receiving a dose of a hemp-derived cannabinoid with higher potency than anticipated.
6) Under the Federal Food, Drug, and Cosmetic Act,7 hemp-derived cannabis products cannot be sold as dietary supplements, food products, or marketed with medical claims. However, beyond issuing a health alert and several warning letters to a small number of companies, there has been little action by the FDA to regulate hemp-derived cannabis products. In the absence of federal regulatory action, 21 states have enacted legislation to restrict or ban the sale of psychotropic, hemp-derived cannabis products.8
However, the sale of psychotropic, hemp-derived cannabis products remains legal in 29 states and in Washington DC, and online sales may render state regulations ineffective.
In conclusion, many of the potential harms of hemp-derived cannabis products stem from a lack of regulation, including the potential for harmful contaminants, accidental exposure, cross-product sale with tobacco and alcohol, and youth appeal. This we believe contributes to the public perception, especially our youth, that all hemp-derived cannabis producs are safe. Government regulators should prioritize new hemp policies that ensure prohibition of sale to minors; set requirements for testing, packaging, and labeling; and place limits on potency and concentration of psychotropic products.
Footnotes
Doctors For Life International is not the author of the above article. This is a shortened version of the original article that can be found here: https://pmc.ncbi.nlm.nih.gov/articles/PMC10406389/
No Conflict of Interest Disclosures were reported
Contributor Information
Alyssa F. Harlow, Department of Population and Public Health Sciences and Institute for Addiction Science, Keck School of Medicine, University of Southern California, Los Angeles..
Adam M. Leventhal, Department of Population and Public Health Sciences and Institute for Addiction Science, Keck School of Medicine, University of Southern California, Los Angeles..
Jessica L. Barrington-Trimis, Department of Population and Public Health Sciences and Institute for Addiction Science, Keck School of Medicine, University of Southern California, Los Angeles..
Doctors for Life International represents more than 1500 medical doctors and specialists, three-quarters of whom practice in South Africa. Since 1991 DFL has been actively promoting sound science in the medical profession and health care that is safe and efficient for all South Africans. For more information visit: http://www.doctorsforlife.co.za
REFERENCES
- 1. Farm Bill. Accessed September 15, 2022. https://www.usda.gov/farmbill
- 2. US Food and Drug Administration. 5 things to know about delta-8 tetrahydrocannabinol-delta-8 THC. Accessed September 16, 2022. https://www.fda.gov/consumers/consumer-updates/5-things-know-about-delta-8-tetrahydrocannabinol-delta-8-thc[PMC free article] [PubMed]
- 3. Leas EC. The hemp loophole: a need to clarify the legality of delta-8-THC and other hemp-derived tetrahydrocannabinol compounds. Am J Public Health. 2021;111(11):1927-1931. doi: 10.2105/AJPH.2021.306499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. US Cannabis Council. The unregulated distribution and sale of consumer products marketed as delta-8 THC. Accessed September 16, 2022. https://irp.cdn-website.com/6531d7ca/files/uploaded/USCC%20Delta-8%20Kit.pdf
- 5. The EVALI outbreak and vaping in the COVID-19 era. Lancet Respir Med. 2020;8(9):831. doi: 10.1016/S2213-2600(20)30360-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levenson MS. Meet THC-O acetate, a hemp-derived compound three times stronger than THC. Published August 19, 2021. Accessed September 16, 2022. https://www.leafly.com/news/cannabis-101/what-is-thc-o[Google Scholar]
- 7. Federal Food, Drug, and Cosmetic Act. Accessed October 31, 2022. https://www.govinfo.gov/content/pkg/COMPS-973/pdf/COMPS-973.pdf
- 8. Cornwell AM. Where is delta-8 THC available or banned? (map of states). Published September 6, 2022. Accessed September 16, 2022. https://cbdoracle.com/news/policy/delta-8-thc-legal/[Google Scholar]
Legalising assisted suicide risks replacing proper care for terminally ill
Doctors for Life and prominent South African doctors are supporting the call to oppose a court challenge by DignitySA to legalise physician-assisted suicide, arguing that dignity “is best upheld through compassionate care and support, enshrined in ubuntu and made possible through the practise of palliative care, rather than through the option of euthanasia”.
Read full article here
COVID-19 Vaccine Informations

COVID-19 Vaccine Candidates and Abortion-Derived Cells Lines

A Visual Aid to Viral Infection and Vaccine Production
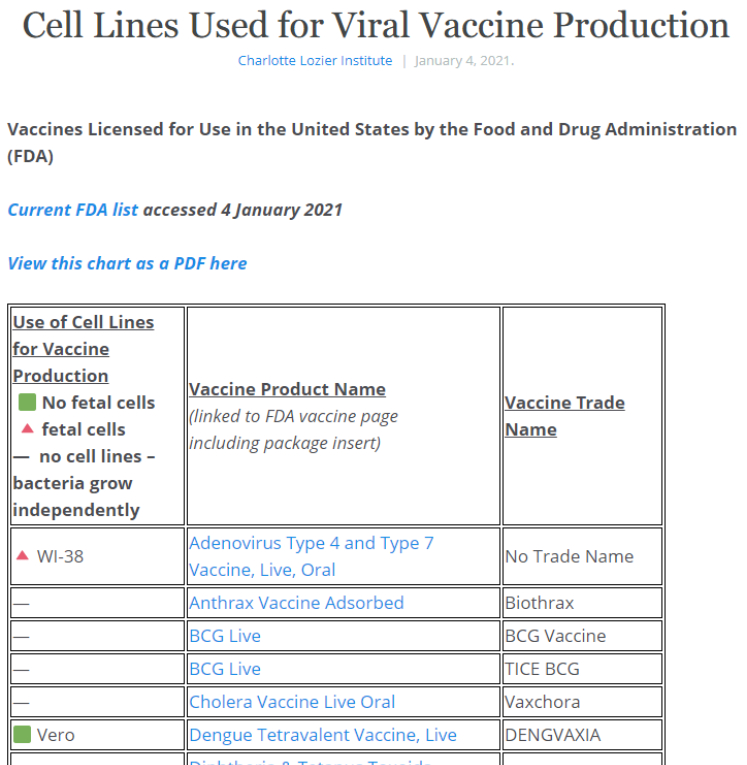
Cell Lines Used for Viral Vaccine Production
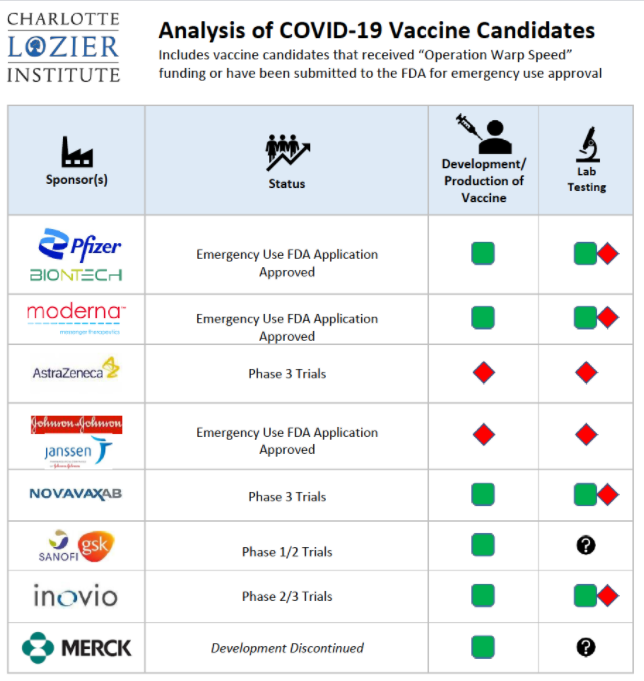
Analysis of COVID-19 Vaccine Candidates